Hydrate: A compound that contains water. Written in the formula: AB x C(H20)
Anhydrous: Without water.
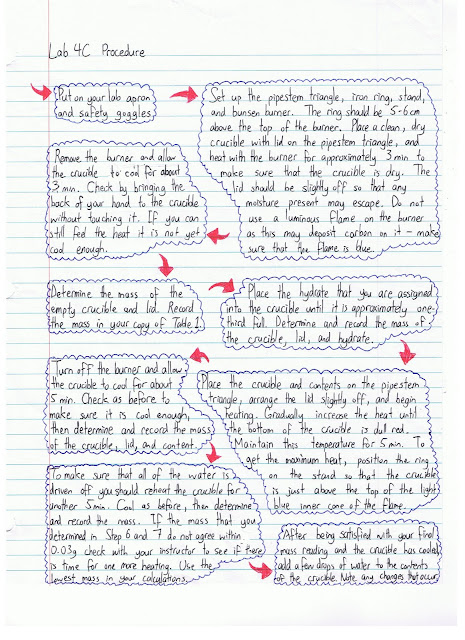
Here is the procedure for our lab:
We found the mass of the water by subtracting the mass of the anhydrous salt after heating from the original hydrate. Then, by dividing the mass of the water by the mass of the hydrate and multiplying by 100, we got a percent of the water in the hydrate. We then determined the number of moles in the salt by dividing the mass of the salt by the molar mass of the hydrate (120.4g). By dividing the mass of the water by the molar mass of the water, we determined the number of moles present in the hydrate.
By: JZ
Showing posts with label lab. Show all posts
Showing posts with label lab. Show all posts
Thursday, December 9, 2010
Wednesday, September 29, 2010
LAB DAY
Today was our first lab day. No one was injured, all was well. We learned how to distinguish between CHEMICAL and PHYSICAL changes.
Please refer to the Tuesday, September 28th, 2010 Blog if you don’t remember their properties. But, if you’re too lazy to scroll down, or you feel insecure with the picture of Jodie’s dog, Bobby, staring at you, here’s a brief definition:
CHEMICAL CHANGE: substances form to create NEW substance(s), irreversible. (e.g. burning wood)
PHYSICAL CHANGE: NO new substance is formed, reversible. (e.g. melting ice cube)
 |
| Glass Square/ Experimental Results Table |
During the lab, we used a set of 4 unknown solutions, and combined them in a 4x4 glass square using medicine droppers. There were only six different combinations. Some had no change, but others showed surprising results.
 |
| Test Tubes |
| Medicine Dropper |
Fun Fact of the Day
DID YOU KNOW… the amount of iron in an adult’s body is equivalent to the amount of iron in an iron nail??
By: Jason Zhang
Subscribe to:
Posts (Atom)