Hydrate: A compound that contains water. Written in the formula: AB x C(H20)
Anhydrous: Without water.
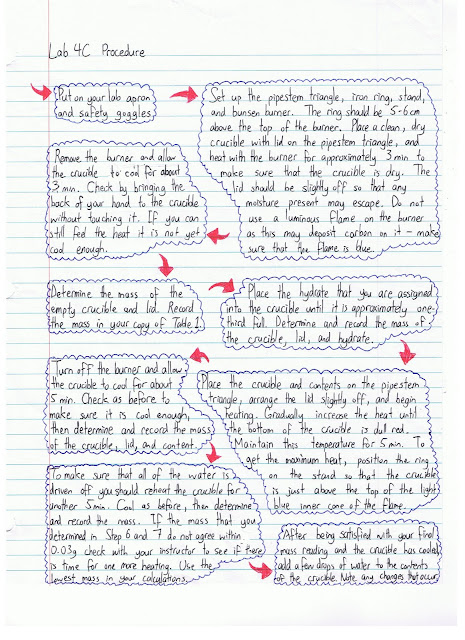
Here is the procedure for our lab:
We found the mass of the water by subtracting the mass of the anhydrous salt after heating from the original hydrate. Then, by dividing the mass of the water by the mass of the hydrate and multiplying by 100, we got a percent of the water in the hydrate. We then determined the number of moles in the salt by dividing the mass of the salt by the molar mass of the hydrate (120.4g). By dividing the mass of the water by the molar mass of the water, we determined the number of moles present in the hydrate.
By: JZ
Thursday, December 9, 2010
Friday, December 3, 2010
Calculating the Empirical Formula of a ORGANIC Compound
CxH2y =(burned in O2)=> x CO2 + y H2O
H: 5.38 / 3.039375 = 1.770100761--> 1.77 x 4 = 7
O: 3.03975 / 3.03975 = 1--> 1 x 4 = 4
ANS: C₅H₇O₄
By: Hikari Hoshika
- From the weight of CO2 and H2O produced by burning a definite amount of the substance, you can figure out the percent of C and H in the compound.
- Percentage of O is usually obtained by subtracting all percentages of C, H, and N, if the compound does not contain any other element.
Review Empirical Formula:
For example:
Calculate the empirical formula of a compound that is 45.99 % C, 5.38% H and the rest is O.
- Find the moles of the compound
C: 45.99g x 1 mol / 12 g = 3.8325 moles
H: 5.38g x 1 mol / 1.0 g = 5.38 moles
O:
- 45.99 + 5.38 = 51.37
- 100 - 51.37 = 48.63 --> O
2. Divide each answer with the lowest number, which is the oxygen.C: 3.8325 / 3.039375 = 1.260950031 --> 1.27 x 4 = 5
H: 5.38 / 3.039375 = 1.770100761--> 1.77 x 4 = 7
O: 3.03975 / 3.03975 = 1--> 1 x 4 = 4
ANS: C₅H₇O₄
By: Hikari Hoshika
Wednesday, December 1, 2010
Empirical & Molecular Formulas!!!
Empirical Formula : help state the lowest ratio of atoms(or moles) inside a formula
*ALL IONIC COMPOUNDS ARE EMPIRICAL FORMULAS*
For example: C12H10 Acnaphthene's empirical formula would be C6H5
To convert molecular formulas to empirical formulas, use this :
1. Convert grams to moles
2. Divide each mole by the lowest molar amount
3. Scale the ratios to whole numbers
An example would be like this:
Calculate the empirical formula of a compound that contains 65% nitrogen and 35% fluorine.
*assume that it adds up to 100 grams*
Step 1: N: 65g x 1 mol = 4.643
14 g
F: 35g x 1 mol = 1.842
19g
Step 2: 4.643 = 2.52
1.842
1.842 = 1
1.842
Step 3: Since 2.52 isn't a whole number, we need to scale it. Multiply each number by a whole number until 2.52 is a whole number .
In this case, 2.52 x 2 = 5.04<----since it's so close you can round down to 5.
Don't forget to multiply 2 to the other number as well . 2 x 1 =2
N5F2 is the empirical formula.
Tip: if the numbers are already whole, just use them as the empirical formula.
Molecular Formula (MF): is the multiple of an empirical formula that shows the actual # of atoms combined to form a molecule.
Formula : n= molar mass of compound
molar mass of empirical formula
Which means that MF= empirical formula x n
Example: A compound contains a empirical formula of C2H3 with a molar mass of 54 g /mol. Find the molecular formular.
Molar mass of C2H3= 27 g/mol
n= 54g/mol =2
27g/mol
MF= 2(C2H3) = C4H6
By : JN
By : JN
Monday, November 29, 2010
Percent Composition
Percent Composition:
The percent composition of a compound is a relative measure of the mass of each different element present in the compound.
To calculate the percent composition (percentage composition) of a compound
The percent composition of a compound is a relative measure of the mass of each different element present in the compound.
To calculate the percent composition (percentage composition) of a compound
- Calculate the molar mass, MM, of the compound
- Calculate the total mass of each element present in the formula of the compound
- Calculate the percent composition: % by weight (mass) of element = total mass of element present ÷ molar mass x 100
Examples:
Calculate the percent by weight of sodium (Na) and chlorine (Cl) in sodium chloride (NaCl)
- Calculate the molecular mass (MM):
- Calculate the total mass of Na present:
- Calculate the percent by weight of Na in NaCl:
- Calculate the total mass of Cl present:
- Calculate the percent by weight of Cl in NaCl:
The answers above are probably correct if %Na + %Cl = 100, that is, 39.34 + 60.66 = 100.
Wednesday, November 24, 2010
HARDER Mole Conversions
By: JZ
 |
| (click to enlarge) |
Conversions between: PARTICLES → MASS
E.g. WHAT IS THE MASS OF: 1.34x1022 Zn atoms?
1.34x1022 atoms X . 1 mole . X 65.4g . = 1.46g of Zn
6.022x1023 1 mole
Conversions between: GRAMS → PARTICLES
E.g. HOW MANY ATOMS WOUD YOU HAVE IF YOU HAVE 10.0g OF COBALT?
10.0g X 1 mole . X 6.022x1023 = 1.02x1023 atoms of Co
58.9g 1 mole
By: JZ
Friday, November 19, 2010
Mole Conversions
Refer to the mole map:
Particles ----> Moles
Ex. How many moles of hydrogen molecules (H2) are present in 9 x 1023 molecules of hydrogen?
1 mole
9.0 x 10²³ molecules of hydrogen X -------------------------------- = 1.5 moles
6.022x10²³ molecules
*** Round to the correct significant figure!
Moles ---> Particles/Molecules/Formula units/atoms
Ex. How many molecules are contained in 3 moles of water molecules, H2O?
6.022x10²³ molecules
3.0 moles X -------------------------------- = 1.8x10²⁴ molecules
1 mole
Moles ---> Grams
Ex. Determine the mass in grams of 3.60 mole of H₂SO₄.
- Find the molar mass of H₂SO₄.
H --> 1.0(2) = 2
S --> 32.1 = 32.1 +
O --> 16.0(4)= 64
---------
98.1 g
2. Solve.
98.1 g
3.60 moles X ------------ = 353g of H₂SO₄
1 mole
Grams ---> Moles
Ex. How many moles of methane molecules, CH4, are in 80 grams of methane?
C --> 12.0 = 12
H --> 1.0(4) = 4 +
------
16 g
1 mole
80g X ------------ = 5.0 moles
16g
By: Hikari Hoshika
Wednesday, November 17, 2010
Who wants some guacamole?
Unfortunately, I was kidding about the title
The mole = volumes of different gases, has a constant ratio.
Amedeo Avogadro
 His law states that equal volume of different gases at the same temperature and pressure have the same number of particles
His law states that equal volume of different gases at the same temperature and pressure have the same number of particles
The mole = volumes of different gases, has a constant ratio.
Amedeo Avogadro
So if the particles are the same then the mass ratio is the mass of the particles.
MOLAR MASS:
- molar mass can be used for atomic/molecular/formula mass and are written in the form of grams per mole (g/mol)
ex. 1 mol of sodium = 23.0
Avogadro's number: 6.022× 1023 PARTICLES
MOLE
Since Ms. Chen mentioned something about a mole day...
not very original don't you think??
Post : JN
Friday, November 5, 2010
To Excel in Excel
Title basically means: to Exceed in Microsoft Excel
(If you didn't get it, it's fine. Not many people understood Kariodisonium-119 either)
Today, we went to the computer lab to learn(?) how to make a graph using Microsoft Excel.
Click here to see what we had to put on the graphs
So here's a video on how to make a graph using Microsoft Excel
By: JZ
(If you didn't get it, it's fine. Not many people understood Kariodisonium-119 either)
Today, we went to the computer lab to learn(?) how to make a graph using Microsoft Excel.
Click here to see what we had to put on the graphs
So here's a video on how to make a graph using Microsoft Excel
By: JZ
Wednesday, November 3, 2010
LAB day. Measuring Thickness of Aluminum Foil
HOW TO FIND THE THICKNESS OF ALUMINUM FOIL
Remember the formula,
density = mass/volume
Also remember the formula, volume = length x width x height. Thickness will substitute height, therefore making the formula, volume = length x width x thickness. Since length x width = area, we could rewrite the density formula to:
density of Al = mass of Al / area of Al x thickness of Al
The formula can be rearranged into:
thickness of Al = mass of Al / area of Al x density of Al
Information needed:
- length and width of aluminum square
- mass measured by a centigram measure
Exponential Error
It expresses the future growth a very small error can become.
Formula:
Exponential error = your measurement - Accepted value / Accepted value x 100 --> %
*It is written as a %
Accepted Value
A value of a substance accepted and approved by many
*given by instructor
By: Hikari H.
By: Hikari H.
Tuesday, November 2, 2010
the knowledge of density can give oneself more knowledge
formula for density is.. Density =Mass
volume
OR
V = m OR m = DV
Density of water is 1000 g/L or 1.0 g/mL
If d of object is < than the d of liquid..then it should be floating on top
otherwise, it would be sinking!
Below is a colourful experiment involving density!
Thursday, October 28, 2010
Accuracy, Precision, and a whole lot of Uncertainty
Accuracy & Precision
What is Accuracy?
- Accuracy is how close a measured value is to the actual (real) value.
What is Precision?
- Precision is how close the measured values are to each other.
For example:
Big black circle: Low Precision
Small Black circle: High Precision
Farther away from Bull's Eye: Low Accuracy
Close to Bull's Eye: High Accuracy
 |
| High Accuracy, High Precison |
 |
| High Accuracy, Low Precision |
 |
| Low Accuracy, High Precision |
Measurement and Uncertainty
No measurement is exact. It is only a best estimate, which has some degree of uncertainty. We only get an exact number when we count a set of objects.
- e.g. there are 23 books on the bookshelf
Absolute Uncertainty
Uncertainty is expressed in the units of measurement, not as a ratio.
To determine absolute uncertainty:
Method 1:
- Discard unreasonable data. Calculate the average of at least 3 measurements. The absolute uncertainty is the largest difference between the average of the highest and the lowest reasonable measurement.
Method 2:
- Determine the uncertainty of each instrument used. Make sure the measurement(s) is as precise as possible. Estimate to 0.1 of the smallest segment on the instrument scale.
e.g. if the smallest measurement on a ruler is in centimetres (cm), measure to 0.1cm, or 1 millimetre (mm)
Relative
Uncertainty = absolute uncertainty / estimated measurement
the ratio can be expressed:
(1) in percent (%)
(2) using significant figures
Here are some videos to help clearify anything you do not understand.
Accuracy and Precision
Measurement and Uncertainty
FUN FACT OF THE DAY:
DID YOU KNOW... each cell in your body has more molecules that there are stars in the Milky Way Galaxy?
By: JZ
Subscribe to:
Posts (Atom)