Hydrate: A compound that contains water. Written in the formula: AB x C(H20)
Anhydrous: Without water.
Here is the procedure for our lab:
We found the mass of the water by subtracting the mass of the anhydrous salt after heating from the original hydrate. Then, by dividing the mass of the water by the mass of the hydrate and multiplying by 100, we got a percent of the water in the hydrate. We then determined the number of moles in the salt by dividing the mass of the salt by the molar mass of the hydrate (120.4g). By dividing the mass of the water by the molar mass of the water, we determined the number of moles present in the hydrate.
By: JZ
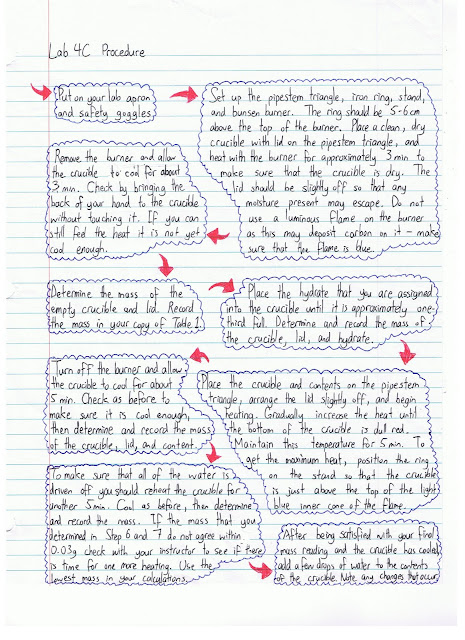
No comments:
Post a Comment