Hydrate: A compound that contains water. Written in the formula: AB x C(H20)
Anhydrous: Without water.
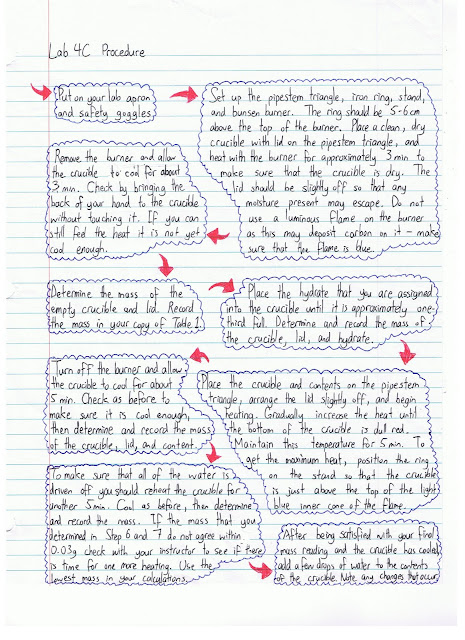
Here is the procedure for our lab:
We found the mass of the water by subtracting the mass of the anhydrous salt after heating from the original hydrate. Then, by dividing the mass of the water by the mass of the hydrate and multiplying by 100, we got a percent of the water in the hydrate. We then determined the number of moles in the salt by dividing the mass of the salt by the molar mass of the hydrate (120.4g). By dividing the mass of the water by the molar mass of the water, we determined the number of moles present in the hydrate.
By: JZ
Thursday, December 9, 2010
Friday, December 3, 2010
Calculating the Empirical Formula of a ORGANIC Compound
CxH2y =(burned in O2)=> x CO2 + y H2O
H: 5.38 / 3.039375 = 1.770100761--> 1.77 x 4 = 7
O: 3.03975 / 3.03975 = 1--> 1 x 4 = 4
ANS: C₅H₇O₄
By: Hikari Hoshika
- From the weight of CO2 and H2O produced by burning a definite amount of the substance, you can figure out the percent of C and H in the compound.
- Percentage of O is usually obtained by subtracting all percentages of C, H, and N, if the compound does not contain any other element.
Review Empirical Formula:
For example:
Calculate the empirical formula of a compound that is 45.99 % C, 5.38% H and the rest is O.
- Find the moles of the compound
C: 45.99g x 1 mol / 12 g = 3.8325 moles
H: 5.38g x 1 mol / 1.0 g = 5.38 moles
O:
- 45.99 + 5.38 = 51.37
- 100 - 51.37 = 48.63 --> O
2. Divide each answer with the lowest number, which is the oxygen.C: 3.8325 / 3.039375 = 1.260950031 --> 1.27 x 4 = 5
H: 5.38 / 3.039375 = 1.770100761--> 1.77 x 4 = 7
O: 3.03975 / 3.03975 = 1--> 1 x 4 = 4
ANS: C₅H₇O₄
By: Hikari Hoshika
Wednesday, December 1, 2010
Empirical & Molecular Formulas!!!
Empirical Formula : help state the lowest ratio of atoms(or moles) inside a formula
*ALL IONIC COMPOUNDS ARE EMPIRICAL FORMULAS*
For example: C12H10 Acnaphthene's empirical formula would be C6H5
To convert molecular formulas to empirical formulas, use this :
1. Convert grams to moles
2. Divide each mole by the lowest molar amount
3. Scale the ratios to whole numbers
An example would be like this:
Calculate the empirical formula of a compound that contains 65% nitrogen and 35% fluorine.
*assume that it adds up to 100 grams*
Step 1: N: 65g x 1 mol = 4.643
14 g
F: 35g x 1 mol = 1.842
19g
Step 2: 4.643 = 2.52
1.842
1.842 = 1
1.842
Step 3: Since 2.52 isn't a whole number, we need to scale it. Multiply each number by a whole number until 2.52 is a whole number .
In this case, 2.52 x 2 = 5.04<----since it's so close you can round down to 5.
Don't forget to multiply 2 to the other number as well . 2 x 1 =2
N5F2 is the empirical formula.
Tip: if the numbers are already whole, just use them as the empirical formula.
Molecular Formula (MF): is the multiple of an empirical formula that shows the actual # of atoms combined to form a molecule.
Formula : n= molar mass of compound
molar mass of empirical formula
Which means that MF= empirical formula x n
Example: A compound contains a empirical formula of C2H3 with a molar mass of 54 g /mol. Find the molecular formular.
Molar mass of C2H3= 27 g/mol
n= 54g/mol =2
27g/mol
MF= 2(C2H3) = C4H6
By : JN
By : JN
Subscribe to:
Comments (Atom)